Developing a Cost-Effective Subject Narratives Solution Using Standards and Automation
Business Challenges
Developing subject narratives from clinical study data is often a manual, tedious, and time-consuming task for medical writers and/or safety specialists. Narratives typically include summary information regarding subject demographics and baseline characteristics, adverse events (AEs) and serious adverse events (SAEs), as well as laboratory values. These data are provided in statistical outputs (eg, tables and listings), which historically are then manually copied/pasted into the narratives. In addition, a list of subjects requiring a narrative must be manually developed based on the study-specific criteria used and the actual narratives generated. Altogether, this results in additional costs, resources, and project time to develop the narratives and then verify their data via quality check (QC) review.
Our client, a large pharmaceutical company, conducted an evaluation of their subject narrative management process and found the following:
- No standards for narrative selection criteria (ie, the reason for which a narrative must be generated)
- No standard format of narratives, or their placement within the clinical study report (CSR)
- No clarity on the QC review process
- No metrics or key performance indicators (KPIs), limiting the ability to do proactive resource planning
Based on these findings, our client wanted to see if there was a way to automate the narratives generation process using a simple-to-use utility that capitalized on existing tools and systems.
The ArborSys Solution
Initially, ArborSys collaborated closely with the client to identify the business requirements for the automated narratives utility. Some of these key requirements included:
- Streamlined, standard format for the narratives, including the corresponding list of subjects requiring a narrative, to address Health Agencies’ requests for easy navigation
- Ability for a user to easily select the required categories for narratives generation
- Auto-population of data into the narratives directly from the statistical outputs
- Ability to collect and capture metrics
- Ability to accurately measure expected resource needs and costs
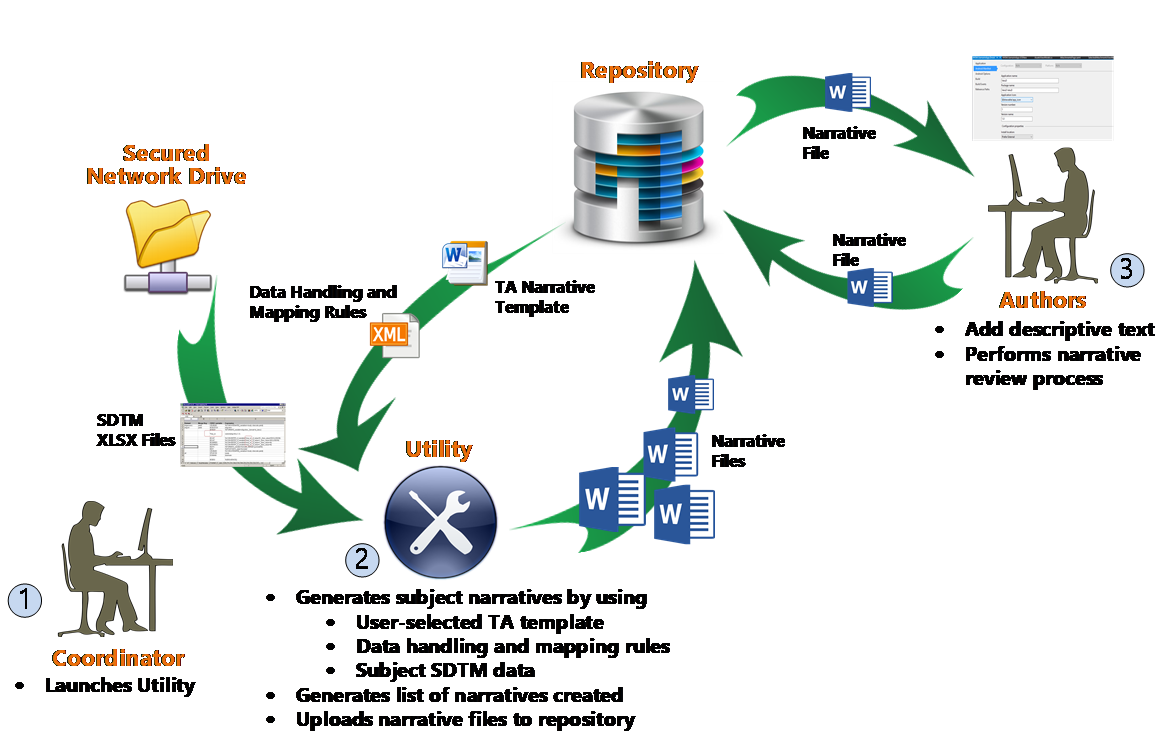
ArborSys worked with the client to define a ‘core’ subject narrative template to use as the starting point for all TAs; this was to ensure consistency in the generated narratives in terms of both format and the baseline data included (eg, demographics and medical history data points). In addition, multiple TA-specific templates were developed based on the ‘core’ requirements, to allow for the automated inclusion of additional data points for a specific TA. Once all the templates’ prespecified data points were identified, ArborSys worked with the client to map where the source data originated from in the CRFs and/or SDTM datasets; the client’s statistical programming group then provided an MS Excel output of the required data in the correct format.
Once all the requirements and data specifications were agreed upon, ArborSys developed an MS Word-based utility that would address these needs by providing the following capabilities:
- To install the utility locally and executed from a user’s desktop
- An intuitive user interface to enable:
- Easy selection of criteria used to determine which subject narratives are generated (eg, subjects who experienced any SAE vs any drug-related SAE)
- Choice of therapeutic area (TA)-specific requirements (eg, the inclusion of summary tables for specific laboratory parameters)
- Ability for the user to identify specific subjects for whom a narrative should be generated on an ad hoc basis
- Utilize TA-specific MS Word narrative templates, which can be updated independently
- Auto-populate pre-identified data points into the MS Word templates from a source data file located on a secured network drive
- The source data file (MS Excel) is aligned with the client’s standard case report forms (CRFs) and SDTM formatted data, to ensure data accuracy and quality
- Data population is determined by a configurable data mapping file, which can be updated at any time
- Generate individual subject narrative files applying standard naming conventions, to facilitate easier compilation downstream
- Generate a comprehensive list of all subjects who had a narrative generated
- Automatically load the narrative files to a central repository (eg, SharePoint) to allow for resource assignments, document lifecycle, and data collection to support KPIs
An overview of the utility’s end-to-end process is illustrated below.
Value Delivered
After production deployment, the new utility immediately yielded positive results such as increased narratives quality, reduced development time, and decreased costs. Initial savings were estimated to range from 30-57% based on the TA; actual savings ranged from 67% for a more complex TA such as oncology, to 25% for a simpler TA such as primary care. Auto-population of prespecified data points reduced the need for downstream data verification and QC review. It also expedited the development of the narrative itself, because the author could focus on writing the narrative text, and not have to tediously find and then copy/paste the required data from the statistical outputs. Utilization of TA-specific templates ensured consistency in the generated narratives, so that teams did not have to “recreate the wheel” each time; standard file naming conventions and a subject list facilitated easy navigation of the narratives within the CSR by Health Agencies’ or other reviewers.
In fact, some time after the initial utility was released, the client asked ArborSys to develop another utility to assist with the compilation of all the individual subject narratives, along with the narratives list, into a single combined document that becomes an appendix of the CSR. This additional utility allowed a user to specify which section in the combined document the narratives should be inserted, and then automatically placed each narrative into its proper section, along with the appropriate bookmarks and hyperlinks, for more efficient PDF publishing as part of the CSR downstream. With the introduction of this supplemental utility, additional savings of 33-45% was realized in the narratives compilation process.
Want to learn more?
For more information about this case study or any of our services, contact us by email: sales@arborsys.com
